Research
Surface Coatings to Inactivate Coronavirus (SARS-CoV-2)
COVID-19 has caused major health concerns and has significantly impacted our everyday lives. The causative agent of the disease is the virus, SARS-CoV-2. Research from other labs has shown that the virus can stay active on solids for several days.1-2 There is potential, therefore, for a person to contract the disease after contacting a surface where the virus was deposited from another person.
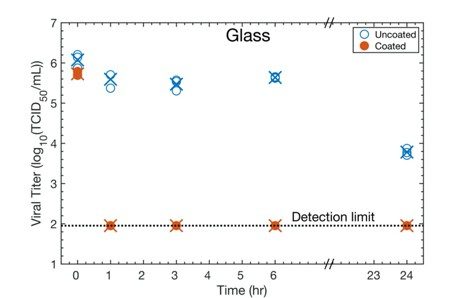
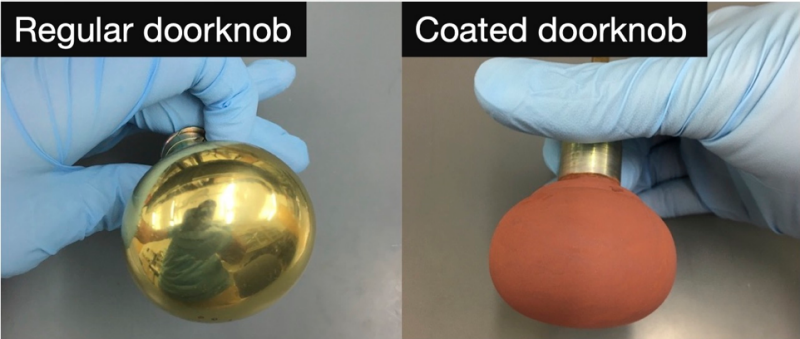
We prepare coatings that rapidly inactivate SARS-CoV-2. The aim is to coat a variety of common-use objects such as doorknobs, shopping cart handles, gas pump handles, and the buttons of a credit card readers (see images below). When respiratory droplets from an individual land on the coated objects then we expect that the virus will be quickly inactivated. The aim is to help to reduce the likelihood of transmission of COVID-19. The coating could also be applied to surfaces in other areas such as hospitals, public transportation, and commercial aeroplane interiors. Our coatings consist of a thin layer of cuprous oxide (Cu2O) on top of a thin layer of commercial polyurethane. In collaboration with Professor Poon’s Laboratory at the University of Hong Kong Medical School, our research shows that the coating inactivates about 99.9% of virus within one hour (See Figure.) This research was published in “A Surface Coating that Rapidly Inactivates SARS-CoV-2”, Behzadinasab, S.; Chin, A, Hosseini, M.; Poon, L; Ducker, W.A., ACS Applied Materials and Interfaces, 2020.
1. Chin, A.; Chu, J.; Perera, M.; Hui, K.; Yen, H.-L.; Chan, M.; Peiris, M.; Poon, L. Stability of SARS-CoV-2 in different environmental conditions. medRxiv 2020.
2. van Doremalen, N.; Bushmaker, T.; Morris, D. H.; Holbrook, M. G.; Gamble, A.; Williamson, B. N.; Tamin, A.; Harcourt, J. L.; Thornburg, N. J.; Gerber, S. I. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. New England Journal of Medicine 2020, 382 (16), 1564-1567.


Adsorption in confined spaces
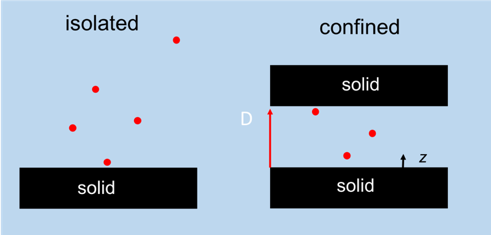
Adsorption of ions, molecules, surfactants, polymers and particles are key to determining the stability of colloidal dispersions and thin wetting films. The forces that lead to stabilization by adsorption often depend primarily on changes to the adsorbed materials as a function of separation between two interfaces. Studies of adsorption overwhelmingly focus on adsorption to isolated surfaces, which is not the same as adsorption in the confined space between two interfaces. Our aim is to directly measure the extent to which adsorption in thin films departs from adsorption in isolated films and to compare measurements to modeling.
Adsorption at Confined Interfaces, Gaddam , P.; Grayson, P. R.; Ducker, W. A., Langmuir, 2018, 34, 10469–10479 https://pubs.acs.org/doi/abs/10.1021/acs.langmuir.8b01418
The Electrostatic Screening-Length in Concentrated Salt Solutions, Gaddam*,, P.; Ducker, W. A. , Langmuir, 2019, 35, 5719–5727.
https://pubs.acs.org/doi/pdf/10.1021/acs.langmuir.9b00375
Bacterial Interactions with Solids: Topography
We are interested in the general problem of how bacteria respond to surface topography when they adsorb. The chief application of this research is to understand how to make materials resist the formation of bacterial biofilms. Biofilms are three-dimensional collections of bacteria, usually coated in a polymer matrix, that adsorb to surfaces. These biofilms form on medical devices, such as catheters, and are a serious health problem. One possible way of delaying bacterial adsorption, surface transport and biofilm formation is to prepare a solid with surface features that are similar to the scale of the bacterium (µm). Compared to chemical treatments, or nanoscale roughness such surfaces cannot be easily “covered up” by adsorption of polymers from solution—the micron scale is just too large. Our expectation is that “topographical surfaces” will have a much longer antibiofilm lifetime or could be used in conjunction with chemical treatments. The important questions are: do they work, and which topography is optimal? In recent work, we have examined whether deposition of colloidal crystals inhibits the attachment of bacteria and the growth into biofilms.
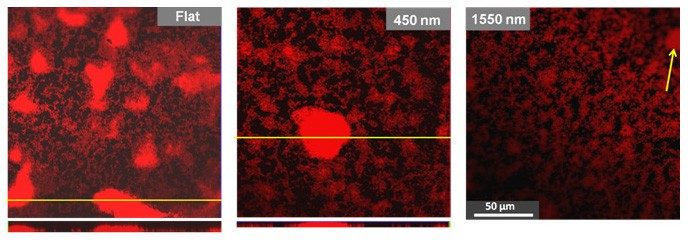
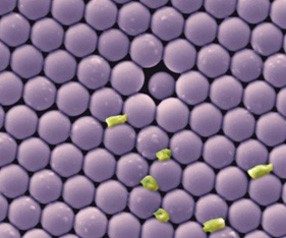
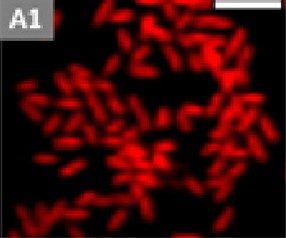
Our research on the bacterium Pseudomonas Aeruginosa shows that:
- (a) fewer bacteria adsorb to colloidal crystals
- (b) the progression to a biofilm is delayed on colloidal crystals.
- (c) the bacteria that do adsorb, do so in the gaps between particles.
Publications on this topic:
Preventing Bacterial Colonization using Colloidal Crystals Kargar, M.; Ducker, W. A. J. Materials Chemistry B., 2014, 2, 5962-5971. DOI 10.1039/C4TB00835A
Antimicrobial Surfaces Using Covalently Bound Polyallylamine, Dmitri D. Iarikov, D. D.; Kargar, M.; Sahari, A.;Russel, L.; Gause, K. T.; Behkam, B.; Ducker, W. A. Biomacromolecules, 2014, 15, 169–176. DOI: 10.1021/bm401440h,
Thermal Rectifiers and Interfacial Resistance
It is well known that, whatever the medium, heat only travels from a hot object to a cold object. However, if the hot and cold object are switched, it is not obvious whether the heat will meet the same resistance going in the opposite direction. If the resistance is different, then the object is called a thermal rectifier, which is analogous to an electrical rectifier, which has a different electric resistance in opposite directions. We are currently performing research to build and understand thermal rectifiers. The main application is heat management. For example, it would be great if a house on a winter day could allow heat in but on a winter night would not allow heat out. Our research in this area also examines heat transfer in thin films.
Publication on this Topic:
The Influence of Interface Bonding on Thermal Transport through Solid–Liquid Interfaces, Harikrishna, H.; Ducker, W. A.; Huxtable, S. T. Applied Physics Letters 2013, 102, 251606.
Recently it has been found that small bubbles exist at the interface between water and hydrophobic solids. We have been investigating the structure, chemistry and stability of these bubbles. The relationship between interfacial and bulk nanobubbles is being studied in collaboration with the company, Revalesio. Our research has focused on trying to determine whether the features really are in the gas state. In the image to the right, the bright spots show tiny air bubbles under water.
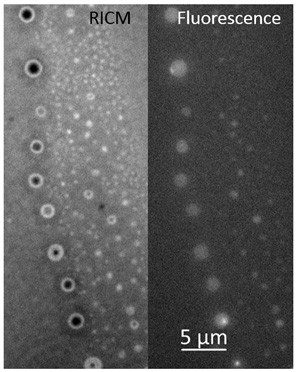
Publications on this Topic:
Phase State of Interfacial Nanobubbles, Seo, D; German, S. R.; Mega, T. L.; Ducker. W.A., Journal of Physical Chemistry C., 2015, 119, 14262-14266.
-
Bio Item
-
General Item
-
General Item
-
General Item
-
General Item







